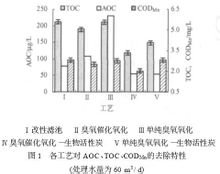
Ozone (O?), also known as superoxide, is an allotrope of oxygen (O?), which is a light blue gas with a special odor at normal temperature. Ozone is mainly distributed in the stratospheric atmosphere at a height of 10 to 50 km, and the maximum value is between 20 and 30 km. In 1840, Germany's CF Scheinen discovered that a special odor gas was released when electrolyzing dilute sulfuric acid, so it was named ozone. Under normal temperature and pressure, the stability is poor, and it can be decomposed into oxygen by itself. Ozone has the taste of grass, and inhalation is beneficial to the human body. Excessive inhalation has certain harm to human health. Non-flammable, pure. Oxygen can be changed to ozone by electric shock. Ozone can be used to purify air, bleach drinking water, sterilize, treat industrial waste and act as a bleaching agent. In the summer, due to the influence of industrial and automobile exhaust gases, especially in the agricultural and forestry areas around large cities, ozone will form and accumulate on the surface. Surface ozone has erosive and damaging effects on the human body, especially on the eyes and respiratory tract. Surface ozone is also harmful to crops or forests. So far, consensus has been reached and a lot of work has been done on the positive effects of ozone and what measures humans should take to protect the ozone layer. However, although the negative effects of the ozone layer have been recognized, there has been no practical and feasible method to solve the problem except for atmospheric monitoring and air pollution forecasting. Basic overview Ozone is a trace gas in the earth's atmosphere. It is formed by the combination of oxygen atoms in the atmosphere and oxygen atoms, which are combined with oxygen molecules in the atmosphere. It contains three oxygen atoms. More than 90% of the ozone in the atmosphere exists in the upper part of the atmosphere or in the stratosphere, 10 to 50 kilometers from the ground. This is the atmospheric ozone layer that needs human protection. There are also a small number of ozone molecules that are close to the ground and still have a role in blocking UV rays. However, some experts have found that the concentration of ozone in the atmosphere near the ground has a tendency to increase rapidly, which is not good. Although ozone plays an important role in protecting humans and the environment in the stratosphere, if it increases in tropospheric concentrations, it will have a detrimental effect on human health. Ozone has a stimulating effect on the eyes and respiratory tract, and also on lung function. Higher concentrations of ozone are also harmful to plants. From the nature of ozone, it can help and harm people. It is both a protective umbrella for heaven and human beings, and sometimes it is like a violent poison. So far, consensus has been reached and a lot of work has been done on the positive effects of ozone and what measures humans should take to protect the ozone layer. However, although the negative effects of the ozone layer have been recognized, there has been no practical and feasible method to solve the problem except for atmospheric monitoring and air pollution forecasting. Discovery process The word "Ozone" comes from the Greek word ozon, which means "sniff". The Spanish name Ozono ozone has an isosceles triangle structure with three oxygen atoms at the three vertices of the triangle and an apex angle of 116.79 degrees. In 1840, Germany's CF Scheinen discovered that a special odor gas was released when electrolyzing dilute sulfuric acid, so it was named ozone. When the oxygen in the atmosphere is photochemically generated, ozone is generated. Therefore, an ozone layer is formed at a vertical height of 15 to 25 kilometers from the ground, and its concentration is 0.2 ppm. The ozone gas is clearly blue, the liquid is dark blue, and the solid is blue-black. Its molecular structure is triangular. Ozone is unstable, decomposes slowly at normal temperature, and decomposes rapidly at 200 ° C. It is more oxidizing than oxygen. It can oxidize metallic silver to silver peroxide, oxidize lead sulfide to lead sulfate, and oxidize organic compounds. If the indigo is ozone, it will discolor. The solubility of ozone in water is greater than that of oxygen. At 0 ° C, a standard volume of water can dissolve 0.494 volumes of ozone. Ozone can irritate mucus membranes, which are toxic to humans and it is not safe to breathe in air containing 0.1 ppm ozone for a long time. The ozone layer can absorb most of the short-wavelength rays (such as ultraviolet rays) and protect humans and other living things. However, chlorine and fluoride promote the decomposition of ozone into oxygen, which destroys the ozone protective layer and becomes one of the important environmental issues of human concern. . Ozone is usually produced from oxygen or air by means of a silent discharge, and the ozone generator is manufactured according to this principle. Using the difference in boiling points of ozone and oxygen, concentrated ozone can be obtained by fractional liquefaction. Ozone is a powerful bleaching agent used to bleach flour and pulp, and to disinfect drinking water with ozone. The water contains only oxygen and no special odor. It is also used for sewage treatment. Ozone is easily decomposed and unstable. It is insoluble in liquid oxygen, carbon tetrachloride and the like. Has a strong oxidizing property, can oxidize silver at normal temperature Silver oxide, which oxidizes lead sulfide to lead sulfate. Ozone decolorizes many organic pigments, erodes rubber, and easily oxidizes organic unsaturated compounds. Ozone is extremely stable in ice and has a half-life of 2000 years. In 1785, when the Germans used the electrodes, they found an odor when the electrodes were discharged. In 1840, the French scientist Christian Frederick identified it as ozone. Under ultraviolet radiation, ozone is naturally formed from diatomic oxygen by electron emission or exposure. Industrially, dry air or oxygen is used to make a silent discharge using an AC voltage of 5 to 25 kV. In addition, ozone can be produced by electrolyzing dilute sulfuric acid at a low temperature or by heating liquid oxygen. Ozone can be used to purify air, bleach drinking water, sterilize, treat industrial waste and act as a bleaching agent. In the summer, due to the influence of industrial and automobile exhaust gases, especially in the agricultural and forestry areas around large cities, ozone will form and accumulate on the surface. Surface ozone has erosive and damaging effects on the human body, especially on the eyes and respiratory tract. Surface ozone is also harmful to crops or forests. Physical and chemical properties Physical properties At normal temperature and normal pressure, the lower concentration of ozone is a colorless gas, and when the concentration reaches 15%, it shows a pale blue color. Ozone is soluble in water. The solubility of ozone in water at normal temperature and normal pressure is about 13 times higher than that of oxygen and 25 times higher than that of air. However, the stability of the aqueous ozone solution is greatly affected by the impurities contained in the water. Especially in the presence of metal ions, the ozone can be rapidly decomposed into oxygen and decomposed slowly in pure water. The density of ozone is 2.14 g/L (0 ° C, 0.1 MP), the boiling point is -111 ° C, and the melting point is -192 ° C. The molecular structure of ozone is unstable, and it is more easily decomposed in water than in air. The main physical properties of ozone are listed in Table 1-1. The solubility of ozone in water at different temperatures is shown in Table 1-2. Although ozone is 10 times more soluble in water than oxygen, its solubility is very small in practice because he follows Henry's law and its solubility is proportional to the partial pressure and total pressure in the system. The content of ozone in the air is extremely low, so the partial pressure is also extremely low, which will force the ozone in the water to escape from the interface between water and air, so that the ozone concentration in the water is always in a decreasing state. Nature data Molecular weight 47.99828 Boiling point -111.9 ° C Melting point -193 ° C Critical temperature -5 ° C Critical pressure 92.3atm Isotonic volume 75.7 (90.2K) Generate heat - 144KJ / mol Solubility in water ml/100ml 49.4 Chemical properties 1. Ozone is very unstable and can be decomposed into oxygen under normal temperature and normal pressure. The oxidation potential (reduction potential) of ozone, chlorine and hydrogen dioxide is 2.07.1.36.1.28 volts, respectively. It can be seen that ozone is the strongest oxidizing power in treated water. Oxidation of ozone results in the breakdown of unsaturated organic molecules. The ozone molecule is bound to the double bond of the organic molecule to form an ozonide. The spontaneous splitting of the ozonide produces a carboxyl compound and a zwitterion with an acidic and basic group which is unstable and decomposes into an acid and an aldehyde. Its reaction formula is: 2O?→3O?+ 285kJ ( 1-2 ) Since a large amount of heat is released during decomposition, when it is more than 25%, it is easy to explode. However, in general, ozone is in the air, and the ozone content is hard to exceed 10%. In the long process of ozone treatment for drinking water, there is no case of an oxygen explosion. The ozone having a content of 1% or less has a decomposition half-life of about 16 hours in an air of normal temperature and normal pressure. As the temperature increases, the decomposition rate increases. When the temperature exceeds 100 ° C, the decomposition is very intense. When the temperature reaches 270 ° C, it can be immediately converted into oxygen. Ozone decomposes faster in water than in air. In an aqueous solution containing impurities, ozone rapidly returns to the oxygen that forms it. For example, when the concentration of ozone in water is 6.25×10 -5 mol/L (3mg/l), the half-life is 5-30min, but the decomposition rate is slower in pure water. For example, the half-life in distilled water or tap water is about 20min ( 20 °C) However, in the double distilled water, the ozone decomposition is only 10% after 85 minutes. If the water temperature is close to 0 °C, the ozone will become more stable. 2. Ozone oxidizing power Ozone has a strong oxidizing power, and its redox potential is second only to F?, which is mainly used in its application. 3. Oxidation of ozone a, oxidation reaction with inorganic substances Ozone reacts with ferrous iron, Mn2+, sulfide, thiocyanide, cyanide, chlorine, etc. Such as: O?+SO?==SO?+O? O?+CO?==CO?+O2 O?+NO?==NO?+O? 3O?+4NH?==NH?NO?+2NH4O? 4O?+PbS==PbSO?+4O? Ag+O?==Ag?O+O? XeO?+4OH?+O?==XeO6+O?+2H?O 3CN-+O?==3OCN? 2OCN?+O?==CO?2?+O?+N? b, the reaction of ozone with organic matter The reaction of ozone with organic matter in aqueous solution is extremely complicated. (1) Reaction of Ozone with Olefin Compounds Ozone is easily reacted with an olefin compound having a double chain, and the final product of the reaction may be a mixture of monomeric, polymeric, or staggered ozonides. Ozone oxides decompose into aldehydes and acids. (2) Reaction of ozone and aromatic compounds The reaction between ozone and aromatic compounds is slow. In the series of benzene <naphthalene < phenanthrene < quinone < quinone < quinone, the reaction rate constant increases gradually. (3) Reacts to nuclear protein (amino acid) and organic ammonia The oxidation sequence of ozone in the following mixtures is Alkenes > Amines > Phenols > Polycyclic Aromatic Hydrocarbons > Alcohols > Aldehydes > Paraffins c, ozone toxicity and corrosivity Ozone is a harmful gas. When the concentration is 6.25×10mol/L (0.3mg/m?), it has a stimulating feeling to the eyes, nose and throat. Concentration (6.25-62.5)×10 -5 mol/L (3~30mg/ When m?), headaches and local paralysis of the respiratory organs occur; when the ozone concentration is 3.125×10 -4 to 1.25×10 -3 mol/L (15 to 60 mg/m?), it is harmful to the human body. The toxicity is also related to the contact time. For example, long-term exposure to ozone below 1.748 × 10 -7 mol / L (4ppm) can cause permanent heart disease, but exposure to ozone below 20ppm does not exceed 2h, no permanent harm to the human body. Therefore, the allowable value of the ozone concentration is set to 4.46 × 10 -9 mol / L (0.1 ppm) 8 h. Since the odor of ozone is very concentrated, the concentration is 4.46 × 10 -9 mol / L (0.1 ppm), people feel Therefore, the use of ozone in the world has been more than 100 years old, and no report of death due to ozone poisoning has been found so far. Ozone is highly oxidizing, and in addition to gold and platinum, ozonized air has a corrosive effect on almost all metals. Aluminum, zinc, lead and ozone are strongly oxidized, but chrome-containing alloys are substantially free of ozone corrosion. Based on this, ferrochrome alloy (stainless steel) containing 25% Cr is often used in production to manufacture parts of ozone generating equipment and filling equipment that are in direct contact with ozone. Ozone also has a strong corrosive effect on non-metallic materials. Even if it is used in a fairly stable polyvinyl chloride plastic filter plate, etc., loose, cracked and perforated are soon used in ozone filling equipment. In the ozone generating equipment and the metering equipment, ordinary rubber cannot be used as the sealing material, and it is necessary to use a silicone rubber or an acid-resistant rubber which is highly resistant to corrosion. Chemical properties and efficacy Ozone (O3) is an allotrope of oxygen (O2), a light blue gas with a distinctive odor. The molecular structure is triangular, the bond angle is 116°, its density is 1.5 times that of oxygen, and its solubility in water is 10 times that of oxygen. Ozone is a strong oxidant. Its redox potential in water is 2.07V, second only to fluorine (2.5V). Its oxidation capacity is higher than chlorine (1.36V) and chlorine dioxide (1.5V), which can destroy decomposing bacteria. The cell wall quickly diffuses into the cell, oxidatively decomposes the glucose oxidase necessary for oxidizing glucose inside the bacteria, and can also directly interact with bacteria and viruses, destroy cells, ribonucleic acid (RNA), and decompose DNA. Macromolecular polymers such as DNA), RNA, proteins, lipids, and polysaccharides destroy the metabolism and reproduction of bacteria. The killing of bacteria by ozone is caused by the breakage of the cell membrane. This process is called cell dissipation. It is caused by the cytoplasm being pulverized in water. It is impossible for cells to regenerate under dissipated conditions. It should be noted that, unlike hypochlorite disinfectants, the bactericidal ability of ozone is not affected by pH changes and ammonia, and its bactericidal ability is 600-3000 times larger than that of chlorine. Its sterilization and disinfection are almost instantaneous. When the ozone concentration in water is 0.3-2 mg/L, the bacteria can be killed within 0.5-1 min. To achieve the same sterilization effect (such as killing E. coli 99%), the amount of ozone water required is only 0.0048% of chlorine. Ozone is also active against yeasts, parasites, etc., for example, it can be used to remove the following types of microorganisms and viruses. 1 The virus has been shown to be very violent to the virus. For example, the Poloi virus loses activity at 2 min when the ozone concentration is 0.05-0.45 mg/L. 2 The cyst was completely removed by a 2.4 min action at an ozone concentration of 0.3 mg/L. 3 spores are 10-15 times more resistant to ozone than the growth bacteria due to the protection of spores. 4 fungi Candida albicans and penicillium can be killed. 5 The parasite, Schistosoma mansoni, was killed after 3 min. In addition, ozone can also oxidize and decompose pollutants in water, and has significant effects on odor removal, decolorization, sterilization, removal of phenol, cyanide, iron, manganese, and reduction of COD and BOD in water treatment. It should be noted that although ozone is a strong oxidant, its oxidizing power is selective, and substances such as ethanol, which are easily oxidized, do not easily interact with ozone. Ozone has strong oxidizing properties. Compared with general UV disinfection, ozone has a strong bactericidal effect. Studies have shown that ozone can kill more than 99% of the killing propagules in 5 minutes. At the same time, ozone also deodorizes. The purpose of many indoor air purifiers is based on the strong oxidizing properties of ozone, the organic matter in the air is oxidized to achieve the purpose of purifying the air. However, the strong oxidizing effect of ozone has a harmful effect on human health. It is generally believed that when ozone is inhaled into the body, it can be rapidly converted into a highly reactive free radical-superoxy (O2-), which mainly oxidizes unsaturated fatty acids, thereby causing Cell damage, formation of cancer. instructions: Ozone is a strong oxidant. The main principle is to stimulate and destroy the deep respiratory mucosa and tissues. It is mildly irritating to the eyes. Prolonged exposure to ozone can damage the central nervous system and cause disorder of thinking. It can also induce lymphocyte chromosome aberration and damage enzymes. Activity and hemolytic reaction, affect thyroid function and cell metabolism in the body, accelerate aging, promote early calcification of bones, etc. Ozone can also cause human neurotoxicity, causing dizziness, headache, decreased vision, memory loss; ozone can also destroy human skin Vitamin E, causing the skin to wrinkle and appear dark spots. Life care: Eat more vegetables on the diet, and the fruits with deep colors Metal Drawers
Looking for metal filing cabinets for sale?
Office Metal Storage Drawers,3 Drawer Metal File Cabinet,Filing Cabinet Price LUOYANG SHIDIU IMPORT AND EXPORT CO., LTD , https://www.shadowcabinete.com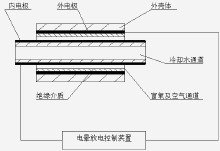




If you need to store heavier material, metal filing cabinets are the way to go.
The two most common forms are Vertical File Cabinet and Lateral File Cabinet.
The office file cabinet on wheels is Mobile Pedestal, which is a small storage unit under desk or office workstations, with 2 or 3 drawer configurations
Staying organized is important in any office environment, whether you are working at home, or in a corporate environment; In every industry, from accountants to architects to designers to health care providers, libraries, retail merchants and more, office furniture are necessary, helping you to organize the documents in order.
From a metal office furniture manufacturer, you always have a full inventory of storage solutions including Metal Cupboards , Metal Lockers , Home Office Storage Cabinets.